by
RICHARD A. NEAL
Bureau of Commercial Fisheries, Biological Laboratory
Galveston, Texas 77550, U.S.A.
Abstract
Methods of staining and tagging shrimp are reviewed. Marking methods discussed are staining by injection, feeding, and immersion, and tagging with internal and external tags. Some aids for handling and releasing live shrimp are also discussed.
METHODES DE MARQUAGE DES CREVETTES
Resumé
Etude du marquage des crevettes par coloration (injection, ingestion et immersion) et l'utilisation de marques internes ou externes. Quelques dispositifs pour manipuler ou relâcher les crevettes vivantes sont également examinés.
METHODS DE MARCADO DE CAMARONES
Extracto
Se pasa reseña de los métodos de coloración y marcado de camarones. Los métodos de marcado discutidos, son los siguientes: coloración por medio de inyección, alimentación e inmersión, así como maroado con maroas internas y externas. Se discuten también los modos más aptos para la manipulación y liberación del camarón vivo.
Several authors have recently reviewed tagging methods used for fish and crustaceans (George, 1967; Rounsefell, 1963; and Arnold, 1966). A number of methods of marking crustaceans have been tested but few are truly satisfactory. The attachment of tags or the injection of foreign materials usually has some undesirable effects.
The problems are numerous in the search for a satisfactory method of marking shrimp. Methods developed for fish usually are not effective for shrimp because of the small size of shrimp, the periodic shedding of the exoskeleton, and the commercial methods of handling and processing shrimp.
The most satisfactory methods of staining, tagging, and handling shrimp are discussed in this paper.
2.1 Development of staining methods
Methods of staining shrimp used first by Menzel (1955) have been refined by several other workers. Dawson (1957) experimented with 26 biological stains as marking agents administered by injection, feeding, and immersion. Costello (1959) injected into shrimp solutions of 65 stains and inks to test their effectiveness as marking agents. Costello and Allen (1961) compared mortality due to predation on stained and unmarked shrimp. They concluded that stained and unstained shrimp were equally vulnerable to predation. Costello (1964) discussed staining techniques, and Klima (1965) evaluated combina ions of several different marks. The techniques for staining shrimp by injection have evolved with the accumulation of experience gained from numerous mark-recapture experiments.
When a biological stain is injected into a shrimp, it accumulates and is retained in the gills (a photograph of a stained shrimp is presented in Fig. 1). If the correct amount of stain is used, it is visible only in the gills about 24 h after staining.
The natural coloring of shrimp either before or after death must be considered in the choice of stains. Red, blue and green are part of the natural coloration of one or more species. The natural colors of the shrimp, the size when stained, and the length of time the mark must remain visible also should be considered in determining the correct amount of stain to be injected. The amount injected should be the smallest amount that will produce a mark satisfactory for a particular study. Any undesirable effects of the stain increase with dosage.
The methods now followed at the Bureau of Commercial Fisheries Biological Laboratory Galveston, Texas, for staining pink shrimp (Penaeus duorarum Burkenroad), brown shrimp (Penaeus aztecus Ives) and white shrimp (Penaeus setiferus Linnaeus) are discussed below.
2.2 Injection procedure
Fresh solutions of stain are mixed before each day's staining because some toxicity may develop in solutions held for more than 1 day (Costello, 1964). Staining solutions are filtered through No. 1 filter paper to remove large particles.
If a quantity of less than 0.1 ml is injected into each shrimp, a small tuberculin syringe with a 6.35-mm (¼-in), 30-gauge needle is used. If more than 0.1 ml is injected, the use of a continuous pipetting outfit equipped with a l-ml Luer-Lok syringe and a 26-or 27-gauge needle facilitates the injection procedure (Fig. 2).
A satisfactory injection site is the articular membrane between the 3rd and 4th abdominal segments. The needle is inserted laterally to minimize injury to the gut, arteries or nerve cord (Fig. 3). Insertion of the needle through the articular membrane minimizes breakage of the exoskeleton and clogging of the needle with bits of exoskeleton.
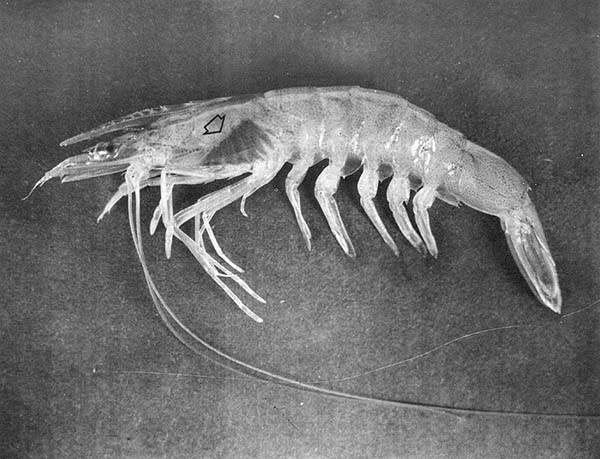
Fig. 1 Penaeid shrimp stained by injection of Niagara sky blue.
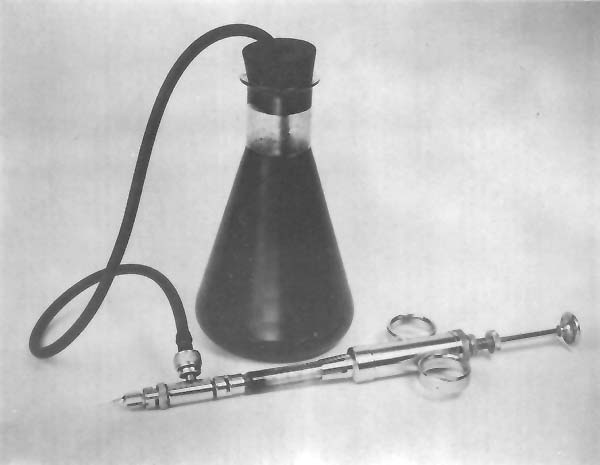
Fig. 2 Continuous pipetting outfit used for rapid injection of biological stains.
2.3 Stains
Fast green FCF mixed with distilled water to form a 0.5% solution of the stain is used for marking shrimp. The amount injected per shrimp ranges from 0.03 to 0.30 ml depending on the size of the shrimp. Because it contrasts sharply with the natural color of shrimp, the green color in the gills is still readily visible after several months. This color fades gradually over a period of 2 to 3 mo so that the vividness of the mark decreases. Nevertheless, it has been used in large-scale field experiments with pink, brown and white shrimp. Recovery of as many as 62% of the shrimp stained and released in these experiments indicates that the adverse effects of the stain are slight, possibly negligible.
Comparison of oxygen uptake of shrimp stained with Fast green FCF with that of unstained shrimp by Zein-Eldin and Klima (1965) disclosed no appreciable difference between the stained and unstained shrimp.
Niagara sky blue 6B in concentrations of 0.25% in distilled water is also used as a shrimp stain. This stain is injected in quantities of 0.03 to 0.30 ml per shrimp. Shrimp retain a vivid blue color in their gills for at least 5 to 6 mo. Shrimp have been stained with Niagara sky blue in field experiments with resulting returns as great as 30% of those released.
Trypan blue likewise has been used successfully in a number of mark-recapture experiments. The color of this stain is retained so well that only small amounts are required to produce a satisfactory mark. When a 0.25% solution of Trypan blue in distilled water is used, 0.03 to 0.15 ml per shrimp is adequate to produce a lasting mark. Costello (1964) demonstrated that Trypan blue and Trypan red are more toxic to pink shrimp than Fast green. The highest percentage of returns of shrimp marked with Trypan blue has been 33%.
Trypan red was used successfully to mark shrimp under laboratory conditions (Dawson, 1957; Costello, 1964). The stain has no apparent value for field studies in the Gulf of Mexico because the natural colors of the shrimp are similar to the color of this stain. Dawson (1957) reported that this stain was clearly visible in the gills of shrimp 8 mo after injection. Quantities of 0.03 to 0.20 ml of a 0.50% solution in distilled water were injected into each shrimp.
Blue and green machine inks injected into shrimp, produced marks satisfactory for short-term studies (Klima, 1965). They faded within 1 week, but apparently did not cause mortality of the shrimp. These inks have not been tested in field experiments.
The sources of stains and marking materials used at the Bureau of Commercial Fisheries Biological Laboratory at Galveston, are identified in Table I.
Dawson (1957) stained small pieces of mullet, Mugil cephalus Linnaeus, with 23 different stains and fed varying quantities to shrimp. Although these feedings caused staining of the anterior digestive tract, some of the stains caused mortality, and fading of the stained area usually was rapid.
The simplicity of staining by immersion and the fact that no physical injury is inflicted upon the shrimp make the possibility of using this method attractive. Results of immersion staining experiments are inadequate, however, to determine if stains are retained through the molting process.
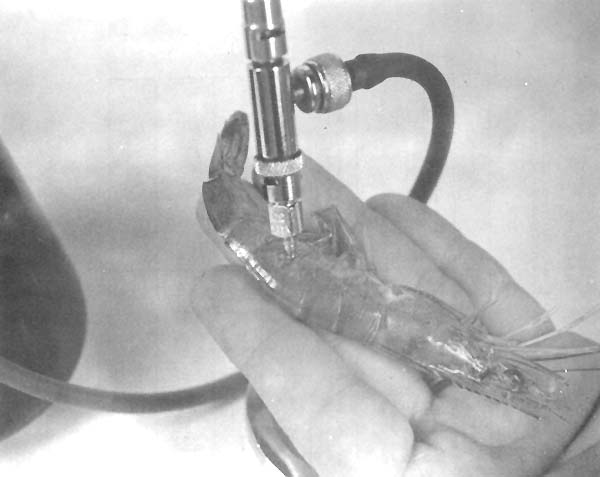
Fig. 3 Injection site for biological stains.
TABLE I
Sources of marking materials used by the Bureau of Commercial Fisheries at the Biological Laboratory, Galveston, Texas1
| Marking material | Source |
| Fast green FCF Niagara sky blue 6B Trypan blue Trypan redi Nile blue A Neutral red | Hartman-Leddon Company Philadelphia, Pennsylvania |
| Blue and green machine inks | Bates Manufacturing Company Orange, New Jersey |
| Red and blue food coloring | French Food Company Rochester, New York |
| Color-coded, wire tags | Technical Research Company Seattle, Washington |
| Day-Glo fluorescent pigments (Saturn yellow, Blaze orange, Arc yellow, and Neon red) | Switzer Brothers, Inc. Cleveland, Ohio |
| Polyvinyl chloride internal tags | Floy Tag Company Seattle, Washington |
1 Trade names referred to in this publication do not imply endorsement of commercial products.
The use of immersion techniques for marking shrimp was discussed by Racek (1956). Of four stains tested, he concluded that two (Trypan blue and Nile blue sulfate) were partially satisfactory. Shrimp stained with Trypan blue had a distinctive nonfading blue tint 2 weeks after immersion. However, about half the shrimp immersed in a solution of Trypan blue for 2 min died after removal from the solution. Racek (1956) stated that Nile blue sulfate was the most promising stain tested. Shrimp were immersed 3 to 4 min in a 0.1% solution of Nile blue sulfate in sea-water. The mark produced was retained for “a long period”.
Dawson (1957) tested Alizarin red S, Bismark brown Y, Janus green B, Methyl green, Nile blue sulfate, Trypan blue, Trypan red, and Fast green FCF to determine their effectiveness as immersion stains. Marks produced by immersion in solutions of all except the last disappeared within 4 days. Immersion of shrimp for 15 min in a 0.75% solution of Fast green FCF in sea water produced some coloration visible up to 6 days after marking.
Immersion staining methods were also examined by Wheeler (1963) who was searching for a method of marking postlarval shrimp. Solutions of ll stains, including liquid food colorings and biological stains, were tested in different concentrations. When shrimp 10 mm in total length (tip of rostrum to tip of telson) were immersed for 10 min in red and blue food colorings, their digestive tract was stained heavily enough so that color was visible 2 to 3 days later, although fading began immediately after their removal from the solution. Immersion of shrimp in solutions of Nile blue A and Neutral red produced staining of the abdominal tissues which was visible for at least 7 and 14 days, respectively.
The need to mark large numbers of Crangon crangon (Linnaeus) led to the examination of various dyes to determine if any were suitable as an immersion stain (Meyer-Waarden and Tiews, 1965). They learned that immersion for 3 to 5 min in a 0.10% solution of methyl violet “gentiana violet B” provided a mark suitable for short-term studies. The stain faded after several weeks, and all traces of the stain disappeared when the animals molted. This method may be of value for studies of shrimp movements or mortality during a single intermolt period.
5.1 Petersen disk tags
Petersen disk tags have been used in a number of studies with several species of shrimp. Extensive studies in which these tags were used were those conducted with white shrimp by Lindner and Anderson (1956). Red and white celluloid disks, individually numbered, 7.9 to 9.5 mm in diameter and 0.25 mm thick were attached to shrimp with a nickel wire pin. One disk was placed on each side of the shrimp, and the pointed end of the pin was bent into a loop so that the disks could not be lost. The pin was inserted through the musculature of the 1st abdominal segment (Fig. 4).
During this study by Lindner and Anderson (1956), the number of deaths resulting from the effects of tagging were higher for small than for large shrimp within the size range 80 to 135 mm total length. Data from returns of this tag were used to determine the migrations and growth rates for this species along the southern Atlantic coast and the Gulf coast of the United States. Of a total of 45,022 shrimp tagged and released, 7,167 or 16% were recovered.
5.2 Wire tags
A silver wire tag with small plastic disks has been used for mark-recapture studies of C. crangon (Kourist, Mauch and Tiews, 1964; Meyer-Waarden and Tiews, 1965, and Tiews, 1967). The wire, 0.18 mm in diameter, is wrapped around the shrimp between the carapace and the 1st abdominal segment. Colored plastic disks 6 mm in diameter are attached to the wire. Some shrimp marked in this way were recovered more than 5 mo after their release and had apparently molted without difficulty while the tag was in place.
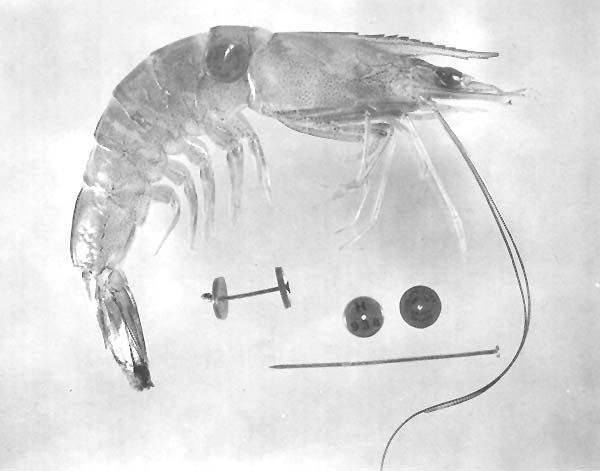
Fig. 4 Shrimp marked with the Petersen disk tag.
5.3 Dart- and loop-type tags
A variety of dart- and loop-type tags have been tested under laboratory conditions at the Galveston Biological Laboratory. These tags were attached to shrimp at several sites on the cephalothorax and abdomen but the junction between the cephalothorax and abdomen was used most frequently. Because the exoskeleton splits at this location during ecdysis, it is a preferred site for tags.
Dart- and loop-type tags which protruded through the exoskeleton frequently caused serious infections. Even though some tagged shrimp survived for several months, the constant irritation prevented healing of the wound, and the infection persisted. Antibiotics applied with the tag did not eliminate infections. These tags may be of value in studies of migration routes, but mortality and growth rates of shrimp are probably affected by the tags.
6.1 Coded-wire tags
A magnetized color-coded wire tag (Jefferts, Bergman and Fiscus, 1963) has been tested as a means of marking Pandalus platyceros Brandt (West, 1967, West and Chew, 1968; and Chew, personal communication). These cylindrical, stainless steel tags 1.0 mm long and 0.25 mm in diameter were injected into the musculature of the 1st abdominal segment. The tagged shrimp were held in aquaria for 50 days without any apparent mortality from tagging. Molting of the tagged shrimp was as frequent as that of untagged controls, and the wounds caused by insertion of the tags healed rapidly. Vertical stripes of colored epoxy paint were applied to wire tags for identification. The numerous possible combinations permit the identification of groups of tagged individuals. An automatic device which injects these tags rapidly into the same position in each animal has been developed by the Technical Research Company. After being inserted into the animals, the tags are magnetized so that they may be detected when the shrimp are passed through an electronic detector.
6.2 Vaseline-pigment marks
In addition to a mark which is easily detected (primary mark), secondary marks may be applied to shrimp to provide a means of identifying individual shrimp or groups of shrimp.
The method of marking with a Vaseline-pigment mixture was developed to aid in recognition of groups of shrimp marked with biological stains (Klima, 1965). Minute quantities (0.003 to 0.010 ml) of mixtures of 1.3-percent Day-Glo fluorescent pigment in Vaseline are injected into the abdominal musculature of the 4th and 5th abdominal segments. Four pigments - Saturn yellow, Blaze orange, Arc yellow and Neon red - can be distinguished readily under ultraviolet light.
Although Klima (1965) stated that the pigment usually remained at the injection site, he observed traces of pigment in the ventral sinuses and gills. Further studies of shrimp marked with the Vaseline-pigment mixture have revealed that particles of the mixture gradually move towards the gill region during a period of several weeks and accumulate in the gills. This movement is particularly apparent if excessive amounts of the Vaseline-pigment mixture are administered.
During the experiments conducted by Klima (1965), the Vaseline-pigment mixture in the bodies of shrimp apparently did not affect their survival. In more recent experiments, however, the mean survival time of shrimp marked with primary and secondary stains was slightly lower than that of unmarked shrimp or of those marked with only a primary stain. The secondary mark has been used by personnel of the Biological Laboratory at Galveston in several field experiments; the percentage returned has been similar for stained shrimp with and without the secondary mark.
6.3 Polyvinyl chloride tags
For identifying individual shrimp marked with a biological stain, personnel at the Biological Laboratory at Galveston developed a small internal tag which can be numbered. This tag is polyvinyl chloride 0.25 mm thick and is about 5 mm by 2 mm (Fig. 5). Slender forceps are used to insert the tag into the musculature of the 1st abdominal segment through the articular membrane joining the carapace with the 1st abdominal segment. The tags are inserted laterally to minimize damage to the nervous, digestive or circulatory systems (Fig. 6). Mortality of tagged and untagged shrimp held in the laboratory was not significantly different.
Polyvinyl chloride tags were used recently in a series of field experiments with brown shrimp. Al shrimp were stained with Niagara sky blue so that they would be recognized by commercial fishermen. In addition, shrimp were marked secondarily with either the polyvinyl chloride tag or a small quantity of a fluorescent Vaseline-pigment mixture as described earlier. About 20,000 marked shrimp were released in three separate experiments during May and June 1967. Of the 13,000 tagged shrimp released, 4.6 percent were returned, and 4.5 percent were returned from the 7,000 marked secondarily with the Vaseline-pigment mixture. Mortalities from marking with this tag apparently were no more severe than those caused by the Vaseline-pigment mark.
7.1 Anesthetics
The vigorous activity of shrimp during handling and marking often results in injuries to the shrimp and loss of time to those doing the marking. Zein-Eldin (1963) conducted tests with sodium pentabarbital, ethclorovynol, methylparafynol, tribromoethanol, chlorobutanol, methol and tricaine methanesulfonate to determine which might be suitable as an anesthetic for penaeid shrimp. Results reported by Zein-Eldin are as follows: “The effective anesthetic does for postlarval shrimp was found in all cases to be at least 10 times greater than that for fish of comparable weight. Moreover, there is a very narrow concentration range in which rapid sedation can be obtained and maintained without fatality.”
7.2 Holding shrimp
Despite reasonable precautions taken to avoid injuring shrimp, some shrimp are injured seriously as a result of handling and marking. In making estimates of rates of fishing mortality, it is desirable to have a reliable estimate of the number of shrimp released. In mark-recapture experiments conducted by the Galveston Laboratory, shrimp are usually held aboard the research vessel for 12 h after marking to minimize post-release mortalities caused by the marking.
Two serious problems that arise when shrimp are held aboard vessels at sea are the turbulence in the holding tanks caused by the constant motion of the vessel, and the fact that water temperatures in holding tanks may be different from the shrimps' natural environment. Because of the turbulence, shrimp are tossed about in the tanks with each sudden movement of the vessel and must swim continuously. To counteract this turbulence, baffles are placed in the holding tanks and irregular surface are provided to which the shrimp may cling. Water being held on the vessel may be warmed by the air and sun, or, if surface water is being pumped into the holding tanks, it may be warmer than the water from which the shrimp were taken. The temperature change to which the shrimp then are subjected along with resulting changes in metabolic rates and oxygen demand may make it impossible to hold live shrimp for any extended period. Refrigeration of the water in the holding tanks to 2° to 5°C below the temperature of the bottom water eliminates this problem and reduces the metabolic rate of the shrimp. This chilling requires, however, the use of a closed system in which the water must be changed regularly if live shrimp are to be held successfully.
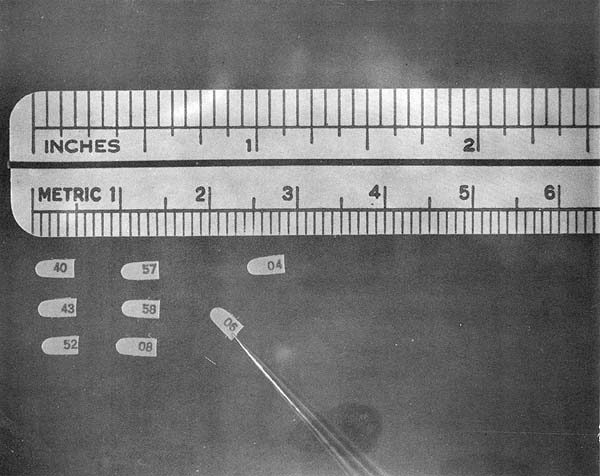
Fig. 5 Internal polyvinyl chloride shrimp tags.

Fig. 6 Method of insertion of internal polyvinyl chloride tags.
7.3 Methods of releasing marked shrimp
Predation at the time of release may cause losses of the marked shrimp. These losses bias mortality estimates based on the number of marked individuals released. Because birds and fish prey upon marked shrimp released at the surface, several types of release boxes have been designed to insure that shrimp reach the botton. The use of these devices increases the shrimps' chances of being able to burrow into the substrate before predators capture them.
Release canisters presently in use at the Biological Laboratory at Galveston are disposable and can be used while the vessel is underway (Benigno and Emiliani, personal communication). They are constructed from resilient styrene sheets and have a release mechanism consisting of a rubber band and salt block. The size of the salt block determines the time elapsing before the shrimp within the box are released (Fig. 7).
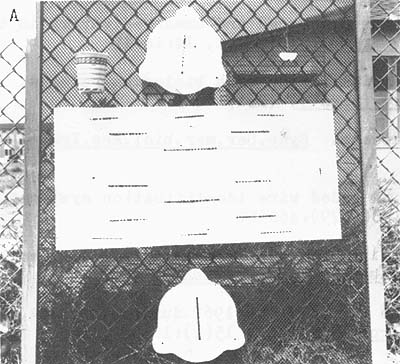 | 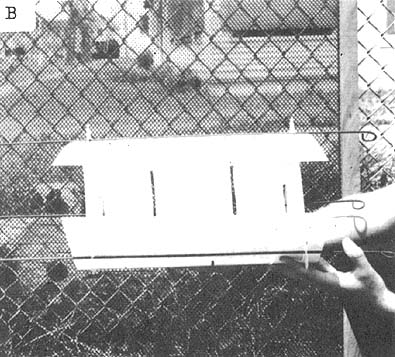 |
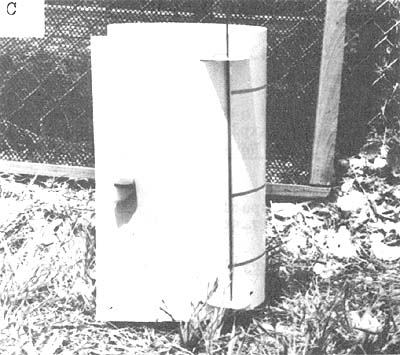 | Fig. 7 Disposable release canister:
|
Because no method of marking shrimp is ideal, the method best suited for each particular study must be chosen by considering the harmful effects of a mark against its advantages. Factors limiting the types of marks which may be used successfully are the behavior of the species, the conditions under which it is being captured and marked, and the specific information being sought.
Several types of marks discussed in this paper have not been used extensively but show promise. Continual refinement of known marking methods and development of new techniques are an important part of shrimp research.
Arnold, D.E., 1966 Marking fish with dyes and other chemicals. Tech.Pap.U.S.Bur.Sport.Fish. Wildl., (10): 3–44
Costello, T.J., 1959 Marking shrimp with biological stains. Proc.Gulf.Caribb.Fish.Inst., 11:1–6
Costello, T.J., 1964 Field techniques for staining-recapture experiments with commercial shrimp. Spec.scient.Rep.U.S.Fish Wildl.Serv.(Fish.), (484):13 p.
Costello, T.J. and D.M. Allen, 1961 Survival of stained, tagged and unmarked shrimp in the presence of predators. Proc.Gulf Caribb.Fish.Inst., 14:16–19
Dawson, C.E., 1957 Studies on the marking of commercial shrimp with biological stains. Spec.scient.Rep.U.S.Fish Wildl.Serv.(Fish.), (231):24 p.
George, M.J., 1967 Mark recovery studies in crustaceans. Symp.Ser.mar.biol.Ass.India, 2(4):1284–95
Jefferts, K.B., P.K. Bergman and H.F. Fiscus, 1963 A coded wire identification system for macro-organisms. Nature, Lond., 198(4879):460–62
Klima, E.F., 1965 Evaluation of biological stains, ink, and fluorescent pigments as marks for shrimp. Spec.scient.Rep.U.S.Fish Wildl.Serv.(Fish.), (511):8 p.
Kourist, W., E. Mauch and K. Tiews, 1964 Ergebnisse von im Jahre 1962 durchgefuhrten Garnelemarkierungsexperimenten. Arch.Fischerei, 15(1):16–22
Lindner, M.J. and W.W. Anderson, 1956 Growth, migrations, spawning and size distribution of shrimp Penaeus setiferus. Fishery Bull.Fish Wildl.Serv.U.S., 56(106):555–645
Menzel, R.W., 1955 Marking of shrimp. Science, N.Y., 121(3143):446 p.
Meyer-Waarden, P.F. and K. Tiews, 1965 Further results of the German shrimp research. Special meeting to consider problems in the exploitation and regulation of fisheries for Crustacea, 1962. Rapp.P.v.Réun.Cons.perm.int.Explor.Mer, 156:131–38
Racek, A.A., 1956 Penaeid prawn fisheries of Australia with special reference to New South Wales. Proc.Indo-Pacif.Fish.Coun., 6(2+3):347–59
Rounsefell, G.A., 1963 Marking fish and invertebrates. Fishery Leafl.Fish Wildl.Serv.U.S., (549):12 p.
Tiews, K., 1967 The use of plastic tags for tagging small shrimps (brown shrimps, Crangon vulgaris Fabricius) and on the problem of tagging experiments of this species of shrimp. Symp.Ser.mar.biol.Ass.India, 2(4):1296–1300
West, W.Q.B., 1967 The use of the Bergman-Jefferts tag on the “spot” shrimp, Pandalus platyceros Brandt. Seattle, Washington, University of Washington, Thesis.
West, W.Q.B. and K.K. Chew, 1968 Application of the Bergman-Jefferts tag on “spot” shrimp, Pandalus platyceros Brandt. Proc.natn.Shellfish.Ass., 58:93–100
Wheeler, R.S., 1963 Immersion staining of postlarval shrimp. In Biological Laboratory, Galveston, Tex., fishery research for the year ending June 30, 1962. Circ.Fish Wildl.Serv., Wash., (161):90–1
Zein-Eldin, Z.P., 1963 Use of anesthetics in metabolism studies with penaeid shrimp. In Biological Laboratory, Galveston, Tex., fishery research for the year ending June 30, 1962. Circ.Fish Wildl.Serv., Wash., (161):63 p.
Zein-Eldin, Z.P. and E.F. Klima, 1965 Effects of injected biological stains on oxygen uptake by shrimp. Trans.Am.Fish.Soc., 94(3):277–8
