L. HUANG and X. ZHOU
Department of Biochemistry and Cellular, Molecular and Developmental
Biology Program
University of Tennessee
Knoxville, TN 37996, USA
Mechanism of delivery
Delivery of plasmid DNA in vitro
Delivery of plasmid DNA in vivo
Conclusions
References
The technique of introducing exogenous DNA into living cells, known as transfection, is a commonly used and very important approach in molecular biology. A number of methods have been developed to achieve this, 1-12 but none of them can likely be used in vivo studies. In this paper we would like to introduce a pH-sensitive immunoliposome delivery system that can efficiently deliver such macromolecules in vivo in a target-specific manner.
Immunoliposomes are lipid vesicles coated with antibodies that have been attached with hydrophobic anchors.13 These surface antibodies direct the specific interaction of liposomes with antigen-expressing cells. Previous studies revealed that the bound vesicles are taken up by cells through a receptor-mediated endocytotic process.14 To prevent liposomes and their contents from being delivered to the lysosomes for degradation, pH-sensitive immunoliposomes are developed to enhance a prelysosomal discharge of the liposome contents into the cellular cytoplasm.
It was found for the first time in this laboratory that liposomes composed of unsaturated phosphatidylethanolamine and a weakly acidic amphiphile, such as fatty acid, become destabilized and fusion-competent when the pH of the medium is reduced to below 6.5.15 If these types of liposomes are endocytosed into endosomes that have a pH range of 5 to 6.5,16,17 they will release their contents into cell cytoplasm by fusing with the endosome membrane from within and/or rupturing the endosome membrane.18 The prelysosomal discharge of the liposome contents significantly enhances the cytoplasmic delivery efficiency of the liposome for anti-tumor drugs,19 toxin20 and DNA.18,21
In our model target system, monoclonal antibodies, anti-H2Kk, are used to direct liposomes to either mouse Ltk or RDM-4 cells that express the murine major histocompatability antigen H-2Kk on the cell surface. Plasmid pPCTK-6A - containing the herpes simplex virus thymidine kinase (tk) gene under control of a cAMP regulatory element, which is located at 5' upstream of the rat phosphoenolpyruvate carboxykinase (PEPCK) gene - were encapsulated into:
1. pH-sensitive immunoliposomes composed of dioleoyl phosphatidylethanolamine P (DOPE):cholesterol: oleic acid (4:4:2), and2. pH-sensitive immunoliposomes composed of dioleoyl phosphatidylcholine (DOPC):cholesterol: oleic acid (4:4:2).
Both types of liposomes were able to deliver the plasmid to target cells as assayed by expression of the gene product (Table 1).22 But the amount of thymidine kinase activity in cells transfected by pH-insensitive immunoliposomes was eight times less than that transfected by pH-sensitive ones. Plasmid DNA entrapped in the pH-sensitive liposomes that bear no antibody also transform the cell phenotype less efficiently. Thus, both pH-sensitivity and liposomal antibody are important for efficient delivery. About 50% of the Ltk cells treated with the pH-sensitive immunoliposomes incorporated H-thymidine into the cellular DNA.
The long-term transformation efficiency of Ltk- cells by pH-sensitive immunoliposomes was also examined and compared with that by traditional calcium-phosphate precipitation method. We arbitrarily defined the "long-term transformation efficiency" as the proportion of treated cells that can undergo at least three cell divisions within 12 days in a selection medium. Therefore, a value of 5.5% was obtained for pH-sensitive immunoliposome method, whereas a much lower efficiency, 6 × 10-4, was obtained for the latter technique.23
To further demonstrate the utility of the pH-sensitive immunoliposome delivery system, a nude mouse model was used to show the target specificity of the DNA delivery. We entrapped into liposomes a plasmid, pBB 0.6, carrying E. coli chloramphenicolacety-1 transferase (CAT) gene under control of PEPCK promoter. The target RDM-4 lymphoma cells bearing H-2Kk antigen were grown as ascites tumor in Balb/c nude mice. A 3H-lipid marker was used to monitor the distribution of liposomes. Several treatments were performed, including:
1) Mice bearing RDM-4 lymphoma cells in peritoneal cavity and injected i.p. with pH-sensitive immunoliposomes containing the plasmid DNA2) As in 1 except that liposomes are pH-insensitive composition
3) As in 1 except that liposomes are antibody-free, and
4) As in 1 except mice bearing no tumor cells
3H distribution in different organs revealed that without the presence of either tumor cells in the mice or antibody on the liposome, the majority of the liposomes were taken up by the spleen, regardless of the lipid composition of the liposomes. Moreover, liposomal antibody was important for targeting; twice the amount of antibody-bearing liposomes was bound to RDM-4 cells compared with the antibody-free liposomes.21
The importance of liposomal antibody and pH-sensitivity for optimal delivery was further confirmed by expression of the CAT gene. CAT activity of RDM-4 cells from mice treated with pH-sensitive immunoliposomes was 6-fold higher than that with antibody-free liposomes. Up to 25-fold increase of CAT activity in the tumor cells was observed when mice were treated with pH-sensitive immunoliposomes instead of pH-sensitive immunoliposomes. Surprisingly, no significant amount of gene product was detected in other organs of the treated mice.21 The organ distribution of the CAT activity in mice treated with the pH-sensitive immunoliposomes containing the plasmid DNA is shown in Figure 1.22 It was evident that the expression of the CAT gene in tumor cells was cAMP-dependent.
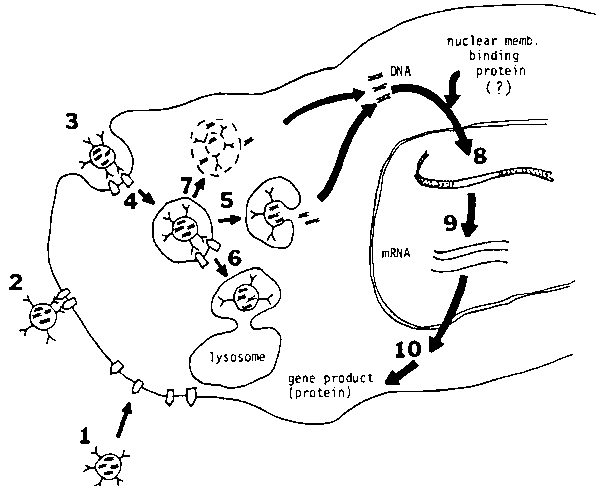
The pH-sensitive immunoliposomes are able to deliver macromolecules specifically to the cytoplasm of the target cell. The working mechanism of this system is summarized in Figure 2.23 The DNA delivery potential of the liposome has been demonstrated both in a tissue culture system and in an animal model. Future work will further develop the liposome system for cancer and viral chemotherapy, vaccine and gene therapy.
1. GRAESSMAN, A., M. GRAESSMAN and C. MULLER. 1979. Curr. Top. Microbiol. and Immunol. 87: 1-21.
2. KLEIN, T.M., E.D. WOLF, R. WU and J.C. SANFORD. 1987. Nature 372: 70-73.
3. GRAHAM, F.L. and A.J. VAN DER EB. 1973. Virol. 52: 456-467.
4. PAGANO, J.S. 1969. In Fundamental Techniques in Virology. K. Habel and N.P. Salzman, Eds.: 184-197. Academic Press, New York.
5. WU, G.Y. and C.H. WU. 1987. J. Biol. Chem. 262: 4429-4432.
6. HAMER, D.H., K.D. SMITH, S.H. BOYER and P. LEDER. 1979. Cell 17: 725-735.
7. STRAWS, S.E. and H.J. RASKA. 1980. J. Gen. Virol 48: 241-245.
8. FRALEY, R., S. SUBRAMANI, P. BERG and D. PAPAHADJOPOULOS. 1980. J. Biol. Chem. 255: 10431-10435.
9. FELGNER, P.L., T.R. GADEK, M. HOLM, R. ROMAN, H.W. CHAN, M. WENZ, J.P. NORTHROP, G.M. RINGOLD and M. DANIELSEN. 1987. Proc. Natl. Acad. Sci. USA. 84: 7413-7417.
10. OKADA, C.Y. and M. RECHSTEINER. 1982. Cell 29: 33-41.
11. FECHHEIMER, M., J.F. BOYLAN, S. PARKER, J.E. SISKEN, G.L. PATEL and S.G. ZIMMER. 1987. Proc. Natl. Acad. Sci. USA 84: 8463-8467.
12. CHU, G., H. HAYAKAWA and P. BERG. 1987. Nucleic Acids Res. 15: 1311-1326.
13. HUANG, A., L. HUANG and S.J. KENNEL. 1980. J. Biol. Chem. 255: 8015-8018.
14. HUANG, A., S.J. KENNEL and L. HUANG. 1983. J. Biol. Chem. 258: 14034-14040.
15. CONNOR, J., M.B. YATVIN and L. HUANG. 1984. Proc. Natl. Acad. Sci. USA 81: 1715-1718.
16. OHKUMA, S. and B. POOLE. 1978. Proc. Natl. Acad. Sci. USA 75: 3327-3331.
17. TYCKO, B. and F.R. MAXFIELD. 1982. Cell 28: 643-651.
18. WANG, C.Y. and L. HUANG. 1987. Biochem. Biophys. Res. Commun. 147: 980-985.
19. CONNOR, J. and L. HUANG. 1986. Cancer Res. 46: 3431-3435.
20. COLLINS, D. and L. HUANG. 1987. Cancer Res. 47: 735-739.
21. WANG, C.Y. and L. HUANG. 1987. Proc. Natl. Acad. Sci. USA 84: 7851-7855.
22. WANG, C.Y. and L. HUANG. 1988. Liposomal DNA delivery: an alternative to the retrovirus-mediated gene therapy? Submitted.
23. WANG, C.Y. 1987. Ph.D. dissertation, University of Tennesee, Knoxville.
Table 1. Effect of liposome composition and targeting ant body on the thymidine kinase activity of Ltk cells
|
Lipid composition |
Antibody |
Activity of tk (cpm/mg protein)a |
|
DOPC:chol:OAb |
+ |
9.7 ± 3.6 |
|
DOPE:chol:OAc |
+ |
76.8 ± 28.7 |
|
DOPE:chol:OAc |
- |
11.7± 6.8 |
a mean s.d. of 30 measurements.
b pH-insensitive composition.
c pH-sensitive composition.
Figure 1. CAT activity in ascites cells and organs of nude mice injected with pH-sensitive immunoliposome containing the plasmid pBB0.6. Mice were also injected with (open bars) or without (hatched bars) a mixture of 8-Br-cAMP and methyl-isobutyl-xanthine to turn on the expression of the CAT gene.
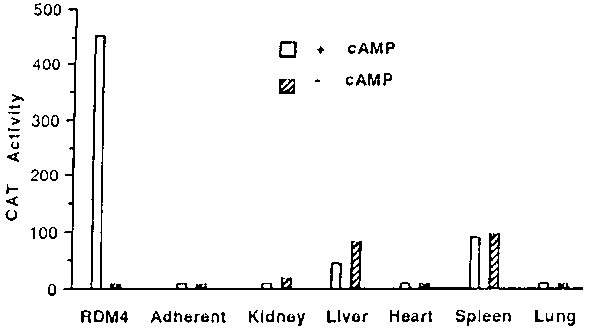
Figure 2. Schematic presentation of the interaction of immunoliposomes with the target cells. The liposomes bind to cell membrane by interaction between antigens ( ) and antibodies (*). 1,2 First, liposomes appear in the endosomes (4) via endocytic pathway. Some of the endocytic liposomes may be transported into lysosomes6 which results in degradation of the liposomal DNA. Most of the pH-sensitive liposomes in the acidic endosomal compartments may fuse with endosomes that result in the delivery of DNA into cytoplasm.5 Alternatively, disruption of the endosomal membrane may also deliver the DNA into cytoplasm.7 The DNA delivered into cytoplasm may be transported into the nucleus by the "nuclear membrane binding protein(s)" or the "DNA-binding protein(s)". The exogenous DNA in the nucleus is eventually integrated into the chromosome8 that results in the expression of gene products.9,10