J.D. LONSDALE-ECCLES, G.W. MPIMBAZA, Y. VERJEE and P. WEBSTER
International Laboratory for Research on Animal Diseases
P O Box 30709, Nairobi, Kenya
Materials and Methods
Results
Discussion
References
A resurgence of interest in proteolytic enzymes has occurred in recent years as a consequence of the greater realization of the importance of proteases in the control of intracellular metabolic processes.1 Their involvement is now known to extend beyond simple protein digestion or the regulation of blood clotting and complement activation, and involves such complex tasks as hormonal processing, intracellular protein transport and the regulation of metabolic pathways. However, most work has concentrated on mammalian cells and only a small amount has been done on microorganisms. For example, our knowledge of the proteolytic enzymes of African trypanosomes is confined almost entirely to those found in the endosomal/lysosomal system, namely, their acidic thiol-dependent proteases.2 In contrast, little or nothing is known about the proteases that are involved in the subtle regulation of their metabolic processes. In part this may be due to the presence of very high levels of lysosomal cathepsin-like activity in the African trypanosomes, which tend to mask the presence of other highly specific enzymes. Consequently, there is a need to find ways of circumventing the blanketing effect of the trypanosomal lysosomal proteases.
In general, proteases are classified according to the mechanism of action of the enzymes. This is frequently ascertained by employing specific inhibitors known to react with particular amino acid residues within the enzymes. For example, trypsin and related enzymes are shown to be serine proteases by their reaction and inhibition with diisopropylfluorophosphate (DFP), a reagent that reacts almost exclusively with serine residues at the active sites of these enzymes. Here we describe some of our observations on the uptake and hydrolysis of fluorescently labelled materials by African trypanosomes as well as some effects of protease inhibitors on these organisms.
Analysis of the thiol-dependent proteolytic activity of African trypanosomes, and of the serum derived modulator of these proteases, was done by electrophoresis in fibrinogen containing sodium dodecylsulphate polyacrylamide gels (Fbg-SDS-PAGE) as described previously.2,4 Detection of serine esterases was performed by incubating 3H DFP (Amersham, UK) with the respective material as described by Scheiner and Quigley.5 The uptake of 3 m M 3H DFP by Trypanosoma brucei6 was performed in pH 8.0 phosphate buffered saline glucose (PSG). Uptake of rhodamine-lisamine-BSA (Molecular Probes Inc., Eugen, OR, USA) was performed on an SLM Aminco 8000 spectrofluorometer (excitation, 560 nm; emission, 590 nm) using trypanosomes isolated6,7 and incubated (in PSG pH 7.4) in the presence of 50 m M hypoxanthine.7 The hydrolysis of Z-Phe-Arg-NHMec was followed specrofluorometrically (excitation, 380 nm; emission, 460 nm).
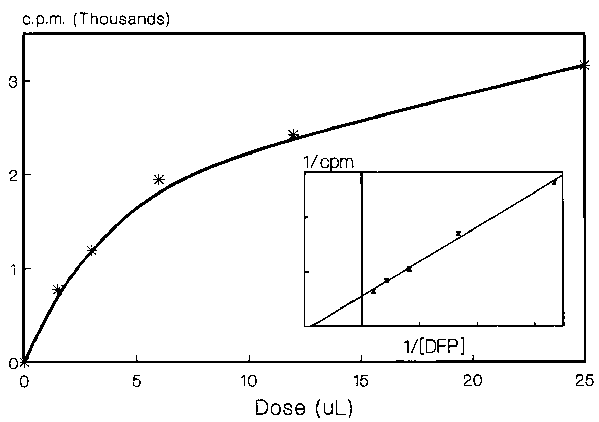
Trypanosoma brucei incorporate 3H DFP into TCA precipitable material. The uptake is dose and time dependent and conforms to a first rate order equation (Figure 1). The chloromethylketones Ac-Ala-Ala-Ala-AlaCK, MeOSuc-ValCK, TPCK, or TLCK had a statistically insignificant effect on the total uptake of 3H DFP by either trypanosomes or trypanosome lysates. Analysis of the proteins labelled with 3H DFP, by SDS-PAGE and fluorography, showed a doublet at 71 kDa, minor bands at 52 and 39 kDa and a band at 27 kDa. Only minor differences were observed between the control and TLCK or TPCK treated samples. Curiously, the presence of TPCK slightly enhanced the labelling of the 39 kDa band. A doublet of Mr approximately 70 kDa was labelled in living trypanosomes, the lower of which was only weakly labelled in homogenates of the parasites.
Analysis by Fbg-SDS-PAGE clearly demonstrates the presence of thiol-dependent proteases in African trypanosomes, which are apparently localized within their endosomal/lysosomal system.2 We have observed and now isolated, a moiety from rat serum which has the capacity to bind to and either stabilize or activate this lysosomal proteolytic activity. The molecule has a similar Mr to serum albumin but, unlike serum albumin, it does not bind to amicon blue dye-A. It also has properties similar to kininogens (pI 4.8) and is found in commercial preparations of human high and low molecular weight kininogens, but again, antibody reactivity suggests distinct identities of the molecules.
Characterization of the activating molecule is under way, but it is also necessary to ascertain whether it is taken up by African trypanosomes. We are therefore studying endocytosis by these parasites. Preliminary studies have been performed using Z-Phe-Arg-NHMec and rhodamine-BSA. Providing the parasites are properly temperature equilibrated, they show an almost immediate steady state hydrolysis of Z-Phe-Arg-NHMec. (If they are not temperature equilibrated there is a lag phase of 1-3 min before a steady state is reached.) The hydrolysis rate of Z-Phe-Arg-NHMec shows a non-linear increase upon increasing the temperature of the reaction medium. The fluorescence of rhodamine-BSA in T. brucei is localized between the nucleus and the flagellar pocket and its uptake is time, dose and temperature dependent.
African trypanosomes contain acid2,8,9,10,11,12 and alkaline2,7,10,13,14 endopeptidase activities. They may also contain cathepsin-D-like activity, although data to support this is weak at present12 (J. Lonsdale-Eccles and G. Mpimbaza, unpublished observations). With respect to the alkaline pH peptidolytic activity we have already shown that trypanosomes contain a DFP and TLCK susceptible activity.2 We wished to see if there were additional serine peptidase activities in the parasites and so tried the method of Scheiner and Quigley5 to explore this possibility. Although we were able to incorporate DFP into discrete proteins, incubation with a variety of chloromethylketones failed to prevent the incorporation of 3H DFP into these molecules. In view of the fact that we have already seen a DFP and TLCK susceptible activity in trypanosomes, it seems unlikely this observation may be the result of the specificity of the enzymes being too tightly defined for the chloromethylketones concerned. It seems more likely that the levels of TLCK and TPCK susceptible serine protease activity in the parasites may be lower than those of the enzymes detected in the SDS gels. Thus, the observed 3H labelled bands in Figure 2 are probably not proteases but rather serine esterases with, as yet, ill-defined specificity. Pertinent to this is the observation that T. brucei contains at least two particle-bound phospholipases which are differentially inhibited by DFP.15
While the situation with respect to serine proteases is still unclear, the situation with respect to the thiol-dependent acid proteases, which we have termed trypanopains, is better defined. One such enzyme has been isolated and purified from T. congolense.11 A similar activity has been located in subcellular fractions containing lysosome-like organelles from T. brucei,2 and in equivalent fractions from T. congolense (J. Lonsdale-Eccles and D. Grab, unpublished observations). Their intralysosomal location would suggest that they play a role similar to that played by the corresponding mammalian intralysosomal activities although this has not yet been delineated. Nor is it clear what controls the activity of these enzymes. However, we have observed that plasma contains a moiety that can bind to and apparently activate or stabilize trypanopain activity when analysed by Fbg-SDS-PAGE.2,4 Curiously, preliminary experiments using Z-Phe-Arg-NHMec showed no apparent enhancement of activity with this moiety. This may mean that the binding of the serum moiety occurs at a regulatory site removed from the active centre of the trypanopain, thereby causing allosteric activation in a manner similar to the La protease. Alternatively, the moiety may stabilize the enzyme against autodegradation or other inactivation processes that may occur during the Fbg-SDS-PAGE analysis.
Because a number of the physical properties of this moiety, such as its pI and Mr. resemble those of low Mr kininogen, we explored this further. Commercial preparations of human kininogens were tested and were found to contain this activity. However, antibody studies suggest that the moiety is distinct from the high or low Mr kininogens. Thus the molecule may be a molecule that is unrelated to kininogens but that which co-purifies with them. Alternatively, it may be an immunologically distinct but related molecule. Other observations suggest that it may be a variant of serum albumin. Although we have not yet identified the molecule, we wish to ascertain whether it is taken up by trypanosomes and regulates their proteolytic activity in vivo. In order to measure the endocytosis of this moiety, we need to set up appropriate endocytotic assays. We have done this by using fluorescently labelled protein that can be monitored quantitatively by spectrofluorometry and qualitatively by microscopy. However, our observations have been complicated by the fact that the uptake of rhodamine-BSA, and other molecules, by trypanosomes in vitro is not always reproducible (J. Lonsdale-Eccles and P. Webster, unpublished observations). Definitive quantitative measurements of endocytosis must therefore await the resolution of this difficulty. Nevertheless, rhodamine-BSA is taken up in a time, temperature and dose dependent manner into a distinct region between the nucleus and flagellar pocket in the area previously suggested to be that in which the endocytotic network is located.16,17 Regardless of its identity, the molecule is a potential candidate to regulate trypanopain activity in vivo and our current studies are aimed at elucidating whether it plays such a role.
1. NEURATH, H. 1986. J. Cell Biochem. 32: 35-49.
2. LONSDALE-ECCLES, J.D. and D.J. GRAB. 1987. Eur. J. Biochem. 169: 467-475.
3. BOND, J.S. and E.P. BUTLER. 1987. Arm. Rev. Biochem. 56: 333-364.
4. LONSDALE-ECCLES, J.D. and G.W.N. MPIMBAZA. 1986. Eur. J. Biochem. 155: 469-473.
5. SCHEINER, C.J. and J.P. QUIGLEY. 1982. Anal. Biochem. 122: 58-69.
6. LANHAM, S.M. and G.D. GODFREY. 1970. Exp. Parasitol. 28: 521-534.
7. LONSDALE-ECCLES, J.D. and D.J. GRAB. 1987. J. Protozool. 34: 405-408.
8. STEIGER, R.F., F. VAN HOOF, F. BONTEMPS, M. NYSSENS-JADIN and J. E. DRUETZ. 1979. Acta Trop. 36: 335-341.
9. STEIGER, R.F., F.R. OPPERDOES and J. BONTEMPS. 1980. Eur. J. Biochem. 105: 335-341.
10. NORTH, M.J., G.H. COOMBS and J.D. BARRY. 1983. Mol. Biochem. Parasitol. 9: 161-180.
11. RAUTENBERG, P., R. SCHADLER, R. REINWALD and H.J. RISSE. 1982. Mol. Cell. Biochem. 47: 151-159.
12. VENKATESAN, S., R.D. BIRD and W.E. OMEROD. 1977. Int. J. Parasitol. 18: 124-193.
13. LETCH, C.A. and W. GIBSON. 1981. Exp. Parasitol., 52: 86-90.
14. KNOWLES, G., S.J. BLACK and D.D. WHITELAW. 1987. Parasitol. 95: 291-300.
15. OPPERDOES, F.R. and J. VAN ROY. 1982. Mol. Biochem. Parasitol. 5: 309-319.
16. LANGRETH, S.G. and A.E. BALBER. 1975. J. Protozool. 42: 40-53.
17. WEBSTER, P. and D.J. GRAB. 1988. J. Cell Biol. 106: 279-288.
Figure 1. Dose dependent uptake of DFP by T. brucei. The parasites (5 × 108) were incubated in 1 mL PSG with various amounts of DFP (1.5 - 25 m L). After 45 min ice-cold TCA was added to give a final concentration of 10% TCA. The precipitate was washed twice with 500 m L 10% TCA and four times with ethanol. The samples were dried and then resuspended in 250 m L SDS-gel sample buffer. 50 m L aliquots were subjected to scintillation counting.