by
H.L. COOK
Bureau of Commercial Fisheries Biological Laboratory
Galveston, Texas 77550, U.S.A.
Abstract
A description is given of a method used to rear penaeid shrimp from eggs to postlarvae in the laboratory. Six species of shrimp have been cultured, and sufficient numbers of Penaeus aztecus Ives and P. setiferus (Linn.) were reared to supply animals for physiological studies and to stock small experimental ponds.
METHODE D'ELEVAGE DE LARVES DE PENAEIDAE EN VUE D'ETUDES EXPERIMENTALES
Résumé
Description de la méthode employée pour élever en laboratoire des Penaeidae depuis l'oeuf jusqu'aux postlarves. L'élevage a porté sur six espèces, et l'on a obtenu un nombre suffisant de Penaeus aztecus Ives et de P. setiferus (Linn.) pour fournir des animaux aux fins d'études physiologiques et pour charger de petits étangs expérimentaux.
METODO DE CULTIVO DE LARVAS DE CAMARONES PENEIDOS PARA ESTUDIOS EXPERIMENTALES
Extracto
Se expone el método empleado para cultivar en laboratorio camarones peneidos a partir de los huevos hasta la fase de postlarvas. Se han cultivado seis especies de camarones, y se criaron cantidades suficientes de Penaeus aztecus Ives y P. setiferus (Linn.) con objeto de proporcionar ejemplares para realizar estudios fisiológicos y para poblar pequeños estanques experimentales.
Research in the United States during recent years on commercially important species of penaeid shrimp has emphasized the importance of the life stages which occur in offshore waters. The latest informational report of the Shrimp Biological Research Committee of the Gulf States Marine Fisheries Commission (1966) states that “Environmental conditions in the nursery areas can affect the abundance of brown shrimp but the principal cause or causes of these annual fluctuations lies in the Gulf of Mexico.” Since almost all penaeid shrimp pass through their larval stages in offshore waters, it is becoming increasingly important to learn more about this stage of their development. Most attempts to study the biology of larval shrimp have been hindered by a lack of larvae with which to work. In fact, the larvae of most species have not even been described.
With the exception of the work of Hudinaga (1942) and Fujinaga (1963) in Japan, nearly all attempts to rear large numbers of penaeid larvae in the laboratory have been unsuccessful. Heldt (1939) and Ewald (1965) succeeded in culturing a few larvae, but the numbers were too small to permit laboratory experimentation. Although Hudinaga was most explicit in his explanation of the methods used to culture larvae of Penaeus japonicus Bate, I have not been able to rear larvae of penaeids from the United States by following his methods.
This paper describes modifications and improvements of the culture techniques described by Cook and Murphy (1966). With this method, sufficient larvae of brown shrimp (P. aztecus) and white shrimp (P. setiferus) have been cultured to supply animals for physiological studies and to stock small experimental ponds. In addition, P. duorarum Burkenroad, Sicyonia brevirostris Stimpson, Trachypenaeus similis (Smith), and Xiphopenaeus kroyeri (Heller) have been reared to postlarvae.
2.1 Field collections
Female shrimp in spawning condition are taken with a commercial 14-m otter trawl. The length of the tow depends on the amount of fish and trash taken along with the shrimp, but is usually between 10 and 30 min. The catch is deposited directly on the deck; if sorted immediately, the ripe females are not hurt by the brief period out of water. On board the ship, the shrimp are held in a tank through which water is circulated continuously. Circulated water is not essential, however, for by changing the water periodically (every 2 h in hot weather), I have held shrimp in containers of various sizes and shapes for several days. The shrimp are transported from the vessel to the laboratory in 75-1 plastic barrels, usually within 24 h of being caught. In extremely hot weather, plastic bags filled with ice are put in the barrels to reduce the temperature. With the temperature reduced in this manner shrimp have been transported more than 120 km with little mortality.
2.2 Laboratory procedures
At the laboratory, the shrimp are placed in 946-1 fiberglass tanks (Fig. 1), described by Marvin (1964), containing 560 1 of water that has been passed through a 5μ cellulose filter. The height of the tanks is 61 cm, inside dimensions are 90 × 152 cm at the bottom, and 110 × 168 cm at the top. Before adult shrimp are placed in the tanks, the sodium salt of EDTA [(Ethylenedinitrilo)-tetracetic acid] is added to the water at a rate of 1 g per 100 1. I have been unable to determine the effect of EDTA, but when it has been added, no unexplained hatching failures or larval mortalities have occurred. On many occasions when EDTA was not used, either the eggs did not hatch, or there were total mortalities of larvae which could not be accounted for. At other times, however, eggs have hatched and larvae have grown to postlarvae in water that did not contain EDTA. At a result, EDTA is added to the water during all phases of the rearing procedure.
As many as 3 shrimp are placed in each tank, depending on the number available. Water in the tanks is recirculated continuously through a crushed oyster shell filter and is aerated vigorously from lead-weighted air stones suspended from the sides of the tank. (The shell contained in the filter is a commercial product processed as a chicken food supplement; it is washed thoroughly before use.) To prevent eggs and larvae from being pumped into the filter, a removable screen is placed over the outlet hose. The screen is a bag made of nylon plankton mesh (0.183-mm mesh opening) attached by elastic bands to a number 11 rubber stopper through which the outlet hose is inserted. A tube of heavy plastic mesh is placed inside the bag to keep it from collapsing. The bag has a surface area of 390 cm2.
The shrimp that spawn usually do so the first night after they are brought to the laboratory. Ordinarily only about one-third of the shrimp produce viable eggs. Some shrimp do not spawn, but resorb their eggs in a few days; others deposit their spawn in a single mass, and the eggs never develop.
After the shrimp spawn, they are removed from the tank to keep them from eating the eggs. Large numbers of larvae have been hatched in the 946-1 tanks, but subsequent survival has been poor. Smaller tanks (90-1 capacity) have also been used satisfactorily for hatching (Cook and Murphy, 1966). Only one large shrimp, however, can be held in each of these tanks, and more tanks must be set up to ensure getting viable eggs.
After the eggs have hatched, a number of nauplii are siphoned from the large tank and concentrated on a stainless steel screen attached to the end of the siphon (Fig. 2c). (The nauplii are hardy and are not injured by such handling if they are kept immersed at all times.) They are then placed in an inverted 19-1 polyurethane carboy from which the bottom has been removed (Fig. 2). Polyurethane is used because it is non-wettable; almost no mortalities are caused by the young shrimp jumping out of the water and becoming attached to the sides of the carboy.
Aeration is supplied by an air stone in the neck of the carboy just above a rubber stopper through which the air hose runs. This arrangement results in good water circulation which, coupled with the sloping shoulders of the carboy, prevents the food from settling out. Each carboy holds 15 l of filtered water, half of which is replaced daily. When water is removed, a screen (Fig. 2c) is placed over the mouth of the siphon to prevent the loss of larvae. As many as 4,000 larvae have been reared to postlarvae in one of these carboys.
2.3 Food
A diatom, Skeletonema costatum (Greville) Cleve, is added as food at the first protozoeal substage. Normally, l l of Skeletonema culture, having an approximate cell count of 2 × 108, is added to each carboy at the first feeding. Thereafter, ½ l is added after each daily water change. Other algae, such as Thallassiosira sp., Dunaliella sp., Gymnodinium splendens Lebour, and Exuviella sp., have also been used successfully as food for the protozoeal substages. When culturing shrimp which have relatively large larvae, such as Penaeus aztecus, newly hatched brine shrimp (Artemia sp.) are supplied at the third protozoeal substage. For species with smaller larvae, such as Trachypenaeus similis, brine shrimp are not added until the first mysis substage. The addition of algae is stopped 1 day after the introduction of brine shrimp.
Although no extensive experiments have been performed to determine the effects of temperature or salinity on larval development, ranges within which larvae have grown well have been delineated. Water temperature should be kept above 24°C. Rate of larval growth increases with increasing temperature up to 32°C, the highest temperature tried. I regard the range from 28° to 30°C as the most favorable. At salinities above 35 and below 27 ‰, hatching has been inhibited and larvae have died. Low-salinity water can be made usable, however, by adding sea salts.
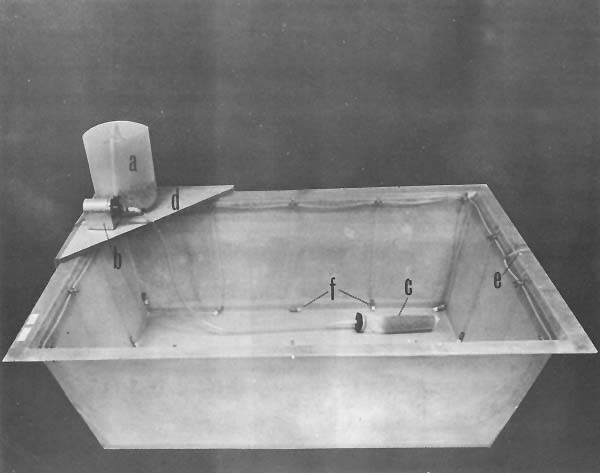
Fig. 1 Fiberglass tank in which shrimp spawn: a, crushed oyster shell filter b, water pump c, screen filter d, support stand e, air hose f, air stone and lead weight

Fig. 2 Polyurethane containers used for culturing larval shrimp: a, support stand b, air hose c, screen assembly used to change water and concentrate larvae
Larval mortality within the inverted carboys has been variable, depending on the initial stocking rate and the amount of food available. In most experiments the estimated survival from Nauplius I to first Postlarva has been about 50 percent. If carboys are stocked with 4,000 nauplii, an average yield of about 2,000 postlarvae per carboy can be expected. Thus, 20,000 postlarvae could be reared with only 10 carboys. This number should be sufficient for most experiments.
Cook, H.L. and M.A. Murphy, 1966 Rearing penaeid shrimp from eggs to postlarvae. Proc. SEast.Ass.Game Fish Commnrs, 19:283–8
Ewald, J.J., 1965 The laboratory rearing of pink shrimp, Penaeus duorarum Burkenroad. Bull. mar.Sci.Gulf Caribb., 15(2):436–49
Fujinaga, M., 1963 Culture of kuruma shrimp (Penaeus japonicus). Curr.Aff.Bull.Indo-Pacif. Fish.Coun., (36):10–1
Gulf States Marine Fisheries Commission. 1966 Shrimp Biological Research Committee, The shrimp fishery of the Gulf of Mexico (Rio Grande River to Key West, Florida). Biological notes and recommendations. Infl.Ser.Gulf St.mar.Fish.Commn, (3):10 p.
Heldt, J.H., 1938 La reproduction chez les crustacés décapodes de la famille des Penéides. Annls Inst.océanogr., Monaco, 18(2):31–206
Hudinaga, M., 1942 Reproduction, development, and rearing of Penaeus japonicus Bate. Jap. J.Zool., 10(2):305–93
Marvin, K.T., 1964 Construction of fibreglass water tanks. Progve Fish Cult., 26(2):91–2