Antimicrobial drugs are essential to treat infectious diseases of humans, animal and plants. However, antimicrobial misuse and overuse in livestock, aquaculture, crop production and human medicine accelerate the emergence and spread of AMR, leading to reduced treatment success or even untreatable infections. Asia and the Pacific region is one of the most impacted regions, with 389 000 people deaths attributed to bacterial AMR in South Asia and 254 000 people deaths attributed to bacterial AMR in Southeast Asia, East Asia and Oceania in 2019 (The Lancet).
FAO ECTAD supports the implementation of the five pillars of the FAO Action Plan on AMR in the region by working towards:
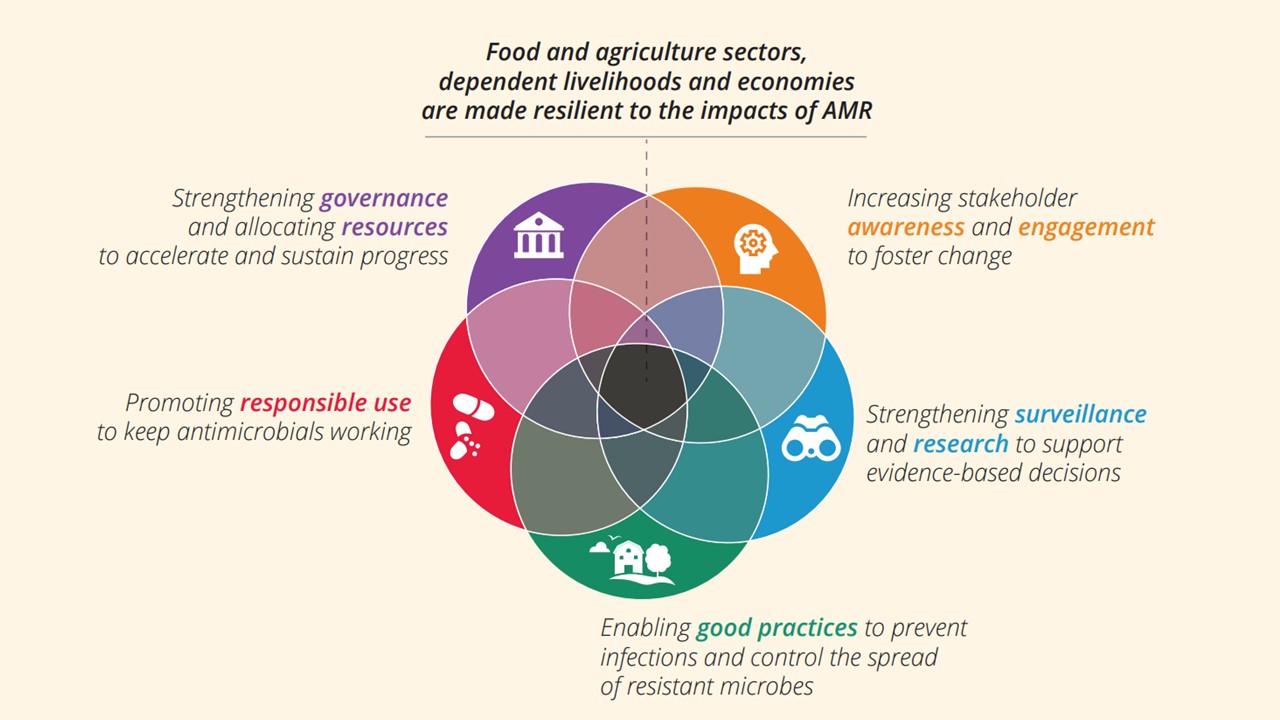
- Enhancing coordination and collaboration among AMR stakeholders through initiating, operationalizing or scaling up multi-sectoral and multi-lateral coordination mechanisms and expanding AMR stakeholder engagement.
- Enhancing institutional and multidisciplinary workforce on AMR through various capacity building initiatives needed to reinforce the overarching objectives of the FAO Action plan in the region.
- Enhancing monitoring and surveillance of AMR, antimicrobial use and antimicrobial residues in animal health systems through strengthening related surveillance system including support to data use for evidence-based interventions.
- Increasing adoption of AMR risk reduction along the value and supply chain, promoting good farming practices and advocating for more prudent antimicrobial use in the animal health sector.
Across these areas, FAO pursues:
- Development of tools: regionally-customized broth microdilution plates, AMR data management templates, regional guidelines on AMR, antimicrobial use and antimicrobial residues monitoring surveillance, WHONET for food and agriculture etc.
- Improvement of systems: implementation of FAO Assessment Tool for Laboratory and AMR Surveillance Systems (ATLASS) and addressing gaps in clinical breakpoints in animals.
- Building of capacities: hands-on regional training on standardized and harmonized methods on antimicrobial susceptibility testing (AST), virtual learning center course on AMR, proficiency testing for AST and AMR surveillance capacity enhancement drive (ASCEnD).
- Access to expertise: setting up of the AMR Technical Advisory Group (TAG), coordination with global FAO reference centre, organizing backstopping missions, FAO and World Organisation for Animal Health (WOAH, formerly OIE) coordinating group of leading AMR institutions in Asia and the Pacific.
- Coordination and partnership: establishment of technical working groups, regional Quadripartite coordination group on AMR, community of practice for veterinary treatment guidelines and private sector engagement.
- Piloting and implementation: pilot AMR monitoring and surveillance and pilot antimicrobial use monitoring at the farm level in select countries.
These are collectively positioned to align with the overarching FAO Action Plan on AMR (2021–2025) which has the goal of leading agrifood systems, dependent livelihoods and economies to become more resilient to the impacts of AMR. These efforts in serving the global public good are made feasible with the strong contributions and invaluable partnerships from countries, collaborators and partners in the region such as Chulalongkorn University (FAO Reference Centre for AMR), Federation of Asian Veterinary Associations (FAVA), Association of Southeast Asian Nations (ASEAN), South Asia Association for Regional Cooperation (SAARC) and many others.

