R.H. DWINGER, A.S. GRIEVE, P. JEANNIN, K. AGYEMANG and J. FAYE
Introduction
Materials and methods
Results
Discussion
References
N'Dama cattle kept under village management conditions and natural trypanosomiasis risk in The Gambia were examined for the presence of anti-trypanosomal antibodies using an indirect immunofluorescent antibody test (IFAT) (Wilson, 1969).
Serological investigations on trypanotolerant cattle using the IFAT have been reported previously for cattle kept under natural trypanosomiasis risk in Liberia (Lucking and Mehlitz, 1978) and in Burkina Faso (Bernard et al., 1980). The IFAT has been successfully used for the diagnosis of cattle trypanosomiasis (Sadun et al., 1963; Wilson et al., 1967). Zwart et al. (1973) reported difficulties in the identification of pure infections with a single trypanosome species and considered the technique of limited use for species differentiation in domestic animals. Toure et al. (1975) also communicated cross-reactions between different trypanosome species to limit the value of the IFAT. However, recent improvements in antigen preparation have made the detection of species-specific antibodies possible (Katende et al., 1987).
The objectives of this study were threefold. Firstly, the time of appearance and the persistence of antibodies was examined in N'Dama cattle bitten by infected tsetse flies under controlled conditions. Secondly, serological investigations of village animals exposed to natural trypanosomiasis risk were performed to provide additional information on the disease status of the animals. This was of particular importance since by direct microscopical examination of blood using the phase-contrast microscopy method (Murray et al., 1977), no low-grade trypanosome infections (less than 200 parasites per ml) can be detected (Paris et al., 1982). Furthermore, chronically infected cattle often show low parasitaemias, especially N'Dama cattle, which are known for their trypanotolerance (Stewart, 1951; Chandler, 1958). Thus, in addition to the regular blood examination of large numbers of village animals, serological techniques were used to detect low-grade or past infections. The third objective was to investigate the time of appearance and duration of anti-trypanosomal antibodies through the monthly collection of serum samples from calves following birth.
Cattle
The N'Dama cattle (Bos taurus) sampled were owned by Gambian farmers and managed under traditional village conditions as described by Dunsmore et al. (1976).
Sampling areas
The herds sampled were kept in two villages situated in different geographical areas with varying tsetse populations. Gunjur is located in a low trypanosomiasis challenge area near the coast where Glossina palpalis gambiensis are present. The village of Keneba is situated 150 km inland in an area moderately infested by G. morsitans submorsitans. In 1986 the annual amount of rainfall in Gunjur was 1135 mm, while in Keneba only 598 mm were measured.
Investigations
Investigation 1
Eighteen male N'Dama cattle of 2-3 years old were selected on the basis of the absence of anti-trypanosomal antibodies. The animals were kept tethered in an area at the coast (Kerr Serigne) believed to be free of tsetse flies and were each fed ad libitum on Andropogon gayanus supplemented with 6 kg daily of a mixture of rice bran, groundnut dust, groundnut cake and salt. Tsetse flies (G. palpalis gambiensis) were captured in a forested area along the Gambia River. Single tsetse flies were transferred to numbered plastic tubes, 6x3 cm in size, with netting on one end. The insects were allowed to feed on fourteen of the experimental animals and following a completed feed were dissected. The proboscis, salivary glands and the gut were microscopically examined for the presence of trypanosomes (Lloyd and Johnson, 1924). Whenever both the gut and the proboscis were found to contain parasites, the tsetse was considered to be infected by T. congolense. If, on the other hand, trypanosomes were found only in the proboscis, the tsetse was considered to be infected by T. vivax. Four of the N'Dama were not bitten by tsetse flies and served as contols.
Investigation 2
A total of about 1000 ear-tagged animals (600 in Gunjur and 400 in Keneba) were sampled monthly for the presence of trypanosomes pathogenic to cattle. From the same animals serum was collected every half year during 1985 and 1986 to investigate the presence of trypanosomal antibodies.
Investigation 3
Following birth, calves were sampled every month to screen their sera for antibodies against trypanosomes pathogenic to cattle. Results were compared with the serological findings obtained for the respective dams in investigation 2.
Sampling techniques
Parasitology
Blood samples were collected monthly (daily in investigation 1) from the jugular vein and put into EDTA-coated vacutainer tubes. These were transported on ice to the laboratory for parasitological examination. Following centrifugation in a micro-haematocrit centrifuge, the buffy coat was examined for trypanosomes using phase-contrast microscopy (Murray et al. 1977).
Serology
Blood samples were collected into plain 10 ml vacutainer tubes every half year (investigation 2), every month (investigation 3) or twice weekly (investigation 1). The samples were placed in a cool box and transported to the laboratory. Following separation by centrifugation, sera were stored at -20°C until testing by IFAT examination.
Indirect immunofluorescent antibody test (IFAT)
Antigens
The antigens used were:
T. congolense ILNat 3.1, a cloned derivative of STIB 212, which was a stabilate prepared after a single passage in rats of an isolate made from a lion in the Serengeti region of Tanzania in 1971 (Geigy and Kauffmann, 1973);T. vivax ILDat 1.3, prepared from Zaria Y486 isolated from a Zebu cow in Nigeria in 1973 (Leeflang et al., 1976); and
T. brucei MITat 1.2, a clone derived from LUMP 427, which was isolated from a sheep in Uganda in 1960 (Cross, 1977).
The trypanosomes were inoculated into rats and following harvesting at peak parasitaemia were separated from the rest of the blood elements and fixed in an acetone/formalin mixture as described by Katende et al. (1987).
Conjugate
Sheep anti-bovine immunoglobulins were prepared using the method described by McGuire et al. (1979) and conjugated to FITC isomer 1 (Sigma, London) using a method similar to that described by Clark and Shepard (1963). The conjugate was stored at 4°C in 1 ml aliquots and after titration was used at a dilution of 1:100.
Bovine lymphocyte lysate
The lysate was prepared according to the method of Goddeeris et al. (1982) and stored at -80°C in 4 ml aliquots.
Test
Initially serum samples were titrated according to the method described by Katende et al. (1987). Later a rapid screening technique was developed by preparing a "cocktail" of the three antigens (N. van Meirvenne, pers. comm.). The sera were screened at a dilution of 1:40. Samples positive to screening with the antigen cocktail were titrated against each of the three antigens in dilutions of 1:40, 1:200, 1:1000 and 1:1500. For each slide, positive and negative control sera diluted at 1:40 were included. The IFAT was otherwise performed as described previously (Katende et al., 1987).
Investigation 1
In order to get one, two or three infected tsetse bites per animal, it was necessary to feed many uninfected tsetse flies on each animal. The number of uninfected complete feeds ranged from five to fifty.
In only five out of the fourteen animals bitten by at least one infected tsetse fly could trypanosomes be detected in the bloodstream by microscopic examination. Two of the infected animals showed a mixed infection of T. vivax and T. congolense, while the remaining three N'Dama had either a T. vivax or a T. congolense infection.
No antibodies against trypanosomes could be detected in the uninfected animals using serological techniques. The time of detection of species-specific antibodies in the infected animals ranged from 15 to 29 days after the infective tsetse bite and followed between 4 and 17 days the initial detection of parasites in the blood. Whenever parasites became non-detectable in the bloodstream, species-specific antibody levels decreased to undetectable levels within two months.
Figure 1. Logarithmic titres of anti-trypanosomal anti-bodies to T. vivax by year of birth (Nov 1985).
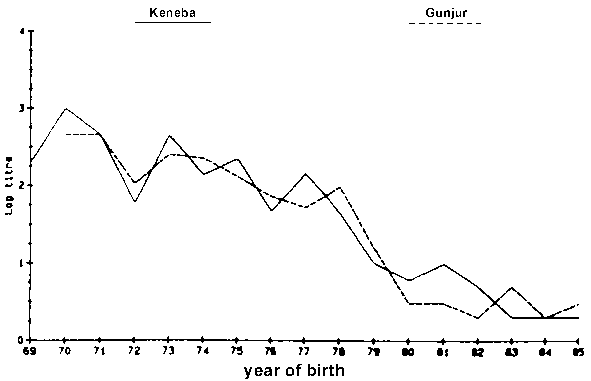
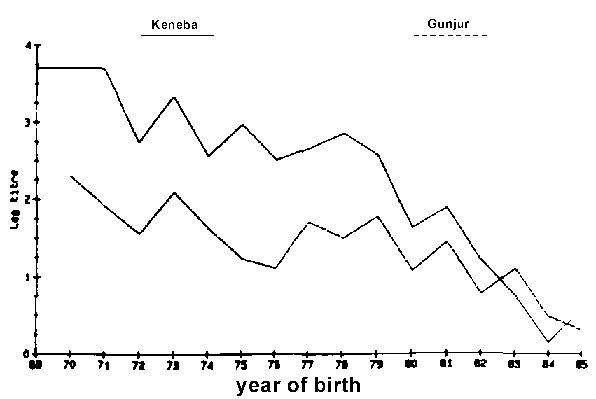
Figure 2. Logarithmic titre of anti-trypanosomal anti-bodies to T. congolense by year of birth (Nov 1985).
Investigation 2
Antibody levels against T. vivax and T. congolense were detected in animals of every age, but older animals had, in general, higher antibody levels (Figures 1 and 2). Only a few animals younger than five years showed any evidence of antibodies against T. vivax. In the case of antibodies directed against T. congolense most of the calves born in 1984 and 1985 were sero-negative (Figures 1 and 2). No antibodies against T. brucei were detected, although antibodies against T. vivax or T. congolense did react with cross-reacting epitomes on the T. brucei antigen but always at lower dilution levels. Different levels of antibody activity were present in the cattle at different times of the year. This was evident when titration levels of antibodies directed against T. congolense in sera collected in November 1985 in Keneba were compared to samples collected in May 1986 and November 1986 (Figures 3, 4 and 5). It appears that within half a year some of the sero-positive animals became sero-negative and that titration levels in others decreased over the same period.
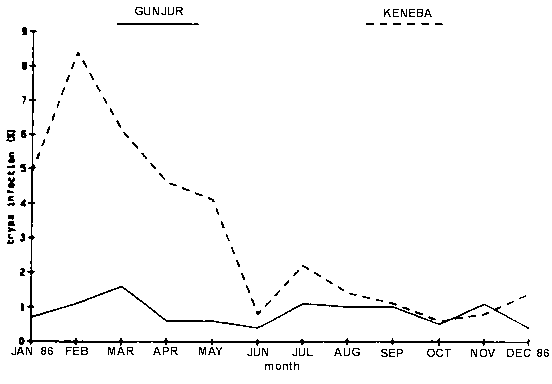
At Gunjur antibody levels against T. congolense were generally lower than at Keneba. However, by November 1986 a large percentage of the animals in each age group in Gunjur and to a lesser degree in Keneba were negative for antibodies against T. congolense. This agrees with the results obtained from microscopical examination of blood samples for parasites: the trypanosome prevalence rate was higher at Keneba than in Gunjur in the beginning of 1986, while both rates dropped during the remainder of the year (Figure 6)
Figure 6. Prevalence of trypanosomiasis in N'Dama cattle by site.
In regard to the percentage of animals possessing antibodies directed against T. vivax there was not a major difference between the Glossina morsitans infested area at Keneba and the Glossina palpalis infested area in Gunjur in November 1985 (Figure 1). However, a difference became apparent during the course of 1986, showing more animals to be sero-negative in Keneba
Investigation 3
Many of the calves were found to possess antibodies against T. congolense and T. vivax during the first few months of their lives (Table 1). No trypanosomes were found in their blood by microscopic examination. On the other hand, the antibody levels correlated very well with the antibody levels in their dams (Table 1). Calves with high levels of antibodies were born out of dams with high antibody levels, while the dams of calves with low antibody levels had low levels. Thus, the antibodies in the young calves were very likely maternally derived. The maternal antibodies disappeared after the third month of age. During the next months of sampling only four calves were found to be positive for T. congolense and none for T. vivax out of a total of 226 calves examined regularly in 1986 and part of 1987 using phase-contrast microscopy. Serologically the infected calves showed only low levels of species-specific antibodies.
Table 1. Presence or absence of antibodies against T. congolense and T. vivax in N'Dama females and their calves in Keneba
|
Trypanosome species |
N'Dama cattle |
High titre |
Low titre |
||
|
Antibody titre |
Number of animals |
Antibody titre |
Number of animals |
||
|
T. congolense |
females |
>1/200 |
14 |
<1/200 |
15 |
|
|
calves |
>1/200 |
13a |
<1/200 |
13b |
|
T. vivax |
females |
>1/200 |
13 |
<1/200 |
16 |
|
|
calves |
>1/200 |
11c |
<1/200 |
14b |
Females were sampled before calving; their calves just after birth and thereafter monthly.
a The remaining calf was sero-negative.
b The remaining 2 calves had titres of >1/200.
c of the remaining 2 calves, one was sero-negative, the other one had a titre of 1/40.
The serological investigations on large numbers of N'Dama cattle in The Gambia indicated that the animals were initially (in November 1985) frequently exposed to tsetse flies infected with T. vivax and/or T. congolense and consequently maintained high levels of antibodies. Continuous surveying of the animals revealed a decrease in antibody levels over time, which was probably due to a decrease in challenge during 1986. This was also reflected in diminished catches of tsetse flies (Snow and Rawlings, pers. comm.) and in a lower number of animals found to be harbouring parasites. Some of the animals found to be sero-positive during one sampling became sero-negative at the next sampling six months later, suggesting that antibody levels were sometimes maintained for less than a half a year. In animals infected under more controlled conditions using tsetse flies caught in the wild, antibodies disappeared even more rapidly once the N'Dama had self-cured. Maternal antibodies were detectable up to three months after birth, which is in agreement with findings by Mehlitz et al. (1983) who detected antibodies at fourteen weeks after birth using an enzyme-linked immunosorbent assay.
It is surprising that the calves found to be positive for parasites on microscopic examination, possessed only low levels of antibodies. As the calves were found to be infected only once, it is likely that they experienced a low-grade infection of too short a duration to elicit high levels of antibodies.
In a large serological survey on trypanosomal antibodies in Zebu and Baoule cattle in Burkina Faso, 87.1% of 519 cattle investigated were found to be serologically positive (titre above 1:20) using an IFAT (Bernard et al., 1980). Only sixty of the animals were trypanotolerant and of those fifty-one (85%) had a titre above 1:20 in the IFAT. In the present survey of the 656 N'Dama examined in The Gambia in November 1985, 320 cattle (48.8%) were found to possess antibodies against T. congolense and 273 animals (41.6%) had antibodies against T. vivax (titres were equal to, or above 1:40).
Desowitz (1959) described high antibody titres in N'Dama born and bred in a tsetse area and attributed the tolerant nature of the N'Dama to maternal antibodies and to continuous challenge. The suggestion that N'Dama cattle can maintain high levels of anti-trypanosomal antibodies for longer periods than zebu (Desowitz, 1959) was confirmed later in other trypanotolerant cattle (Akol et al., 1986). On the other hand, tolerant animals were not found to have a more effective antibody response than sensitive zebu cattle following experimental infection with a T. brucei brucei clone (Pinder et al., 1984). In conclusion, although the mechanism of trypanotolerance in N'Dama cattle is still largely unresolved (Roelants et al., 1983; Murray et al., 1982), this study on anti-trypanosomal antibodies in trypanotolerant animals indicates that once the size of the trypanosome population has decreased to undetectable levels, antibodies disappear unless re-infection occurs. Moreover, maternal antibodies were found to be passed on to offspring and to persist for a period of three months. Finally, monthly sampling was found to give a more accurate impression of changes in antibody levels in village animals kept under natural trypanosomiasis risk than half-yearly sampling due to the short persistence of species-specific antibodies.
Acknowledgements
We are grateful to Dr. J.M. Katende for the provision of reagents used in the IFAT, Professor N. van Meirvenne for valuable technical advice and Dr. P. Rawlings for catching and dissecting tsetse flies. The availability of the facilities of ITC and the encouragement by the centre's directors are greatly appreciated.
Akol, G.W.O., E. Authie, M. Pinder, S.K. Moloo, G.E. Roelants and M. Murray. 1986. Susceptibility and immune response of zebu and taurine cattle of West Africa to infection with Trypanosoma congolense transmitted by Glossina morsitans centralist Vet. Immunol. Immunopath. 11: 361-373.
Bernard S., M. Hasse and G. Guidot. 1980. Trypanosomose der Rinder in Obervolta, epizootologische Ermittlungen und Beitrag zum Problem Trypanotolernz. II. Enzym-immuntest und indirekte Immunofluoreszenz zum Nachweis von Antikorpern gegen Trypanosomen bei trypanotoleranten und trypanosensiblen Rindern. Berl. Munch. Tierarztl. Wschr. 93: 482-485.
Chandler, R.L. 1958. Studies on the tolerance of N'Dama cattle to trypanosomiasis. J. Comb. Path. 68: 253-260.
Clark, H.F. and C.C. Shepard 1963. A dialysis technique for preparing fluorescent antibody. Virology, 20: 642-644.
Cross, G.A.M. 1977. Antigenic variation in trypanosomes. Am. J. trop. Med. Hyg. 26: 240-244.
Desowitz, R.S. 1959. Studies on immunity and host-parasite relationships I. The immunological response of resistant and susceptible breeds of cattle to trypanosomal challenge. Ann. Trop. Med. Parasitol. 53: 293-313.
Dunsmore, J.R., A. Blair Rains, G.D.N. Lowe, D.J. Moffatt, I.P. Anderson and J.B. Williams. 1976. The agricultural development of The Gambia: an agricultural, environmental and socioeconomic analysis. Land resource study 22. Ministry of Overseas Development, Great Britain. pp. 224-230.
Geigy, R. and M. Kauffmann. 1973. Sleeping sickness survey in the Serengeti area (Tanzania) 1971. Part I. Examination of large mammals for trypanosomes. Acta trop. 30: 12-23.
Goddeeris, B.M., J.M. Katende, A.D. Irvin and R.S.C. Chumo. 1982. The indirect fluorescent antibody test for experimental and epizootiological studies on East Coast Fever (Theileria parva infection in cattle). Evaluation of a cell culture schizont antigen fixed and stored in suspension. Res. vet. Sci. 33: 360-365.
Katende J.M., A.J. Musoke, V.M. Nantulya and B.M. Goddeeris. 1987. A new method for fixation and preservation of trypanosomal antigen for use in the indirect immunofluorescence antibody test for diagnosis of bovine trypanosomiasis. Trop. Med. Parasit. 38: 41-44.
Leeflang, P. A., J. Buys and C. Blotkamps 1976. Studies on Trypanosoma vivax: infectivity and serial maintenance of natural bovine isolates in mice. Int. J. Parasitol. 6: 413-417.
Lloyd L. and W.B. Johnson. 1924. The trypanosome infections of tsetse flies in Northern Nigeria and a new method of estimation. Bull. ent. Res. 14: 265-288.
Luckins, A.G. and D. Mehlitz. 1978. Evaluation of an indirect fluorescent antibody test, enzyme-linked immunosorbent assay and quantification of immunoglobulins in the diagnosis of bovine trypanosomiasis. Trop. Anim. Hlth Prod. 10: 149-159.
McGuire T.C., A.J. Musoke and T. Kurtti. 1979. Functional properties of bovine IgG1 and IgG2: Interaction with complement, macrophages, neutrophils and skin. Immunology 38: 249-256.
Mehlitz, D., S. Heidrich-Joswig, H.-O. Fimmen, E.K. Freitas and E. Karbe. 1983. Observations on the colostral transfer of anti-trypanosome antibodies in N'Dama calves and the immune response to infection with Trypanosoma (Duttonella) vivax and T. (Nannomonas) congolense. Ann. Soc. beige Med. trop. 63: 137-148.
Murray, M., W.I. Morrison and D.D. Whitelaw. 1982. Host susceptibility to African trypanosomiasis: trypanotolerance. Adv. Parasitol. 21: 1-68.
Murray, M., P.K. Murray and W.I.M. McIntyre. 1977. An improved parasitological technique for the diagnosis of African trypanosomiasis. Trans. roy. Soc. trop. Med. Hyg. 71: 325-326.
Paris, J., M. Murray and F. McOdimba. 1982. A comparative evaluation of the parasitological techniques currently available for the diagnosis of African trypanosomiasis in cattle. Acta trop. 39: 307-316.
Pinder, M., G. Libeau, W. Hirsch, I. Tamboura, R. Hauck-Bauer and G.E. Roelants. 1984. Anti-trypanosome specific immune responses in bovids of differing susceptibility to African trypanosomiasis. Immunology 51: 247-258.
Roelants, G.E., I. Tamboura, D.B. Sidiki, A. Bassinga and M. Pinder. 1983. Trypanotolerance. An individual not a breed character. Acta trop. 40: 99-104.
Sadun, E.H., R.E. Duxbury, J.S. Williams and R.I. Anderson. 1963. Fluorescent antibody test for the serodiagnosis of African and American trypanosomiasis in man. J. Parasitol. 49: 385-388.
Stewart, J.L. 1951. The West African Shorthorn cattle. Their value to Africa as trypanosomiasis-resistant animals. Vet. Rec. 63: 454-457.
Toure, S.M., M. Seydi, M. Seye and B. Rebel 1975. Valeur de la methode d'immunofluorescence indirecte dans le diagnostic des trypanosomiasis bovines et leur etude epizootiologique. Rev Elev. Med. vet. Pays trop. 28: 463-472.
Wilson, A.J. 1969. Value of the indirect fluorescent antibody test as a serological aid to diagnosis of Glossina-transmitted bovine trypanosomiasis. Trop. Anim. Hlth Prod. 1: 89-95.
Wilson, A.J., M.P. Cunningham and C.D. Kimber. 1967. The indirect fluorescent antibody test applied to bovine trypanosomiasis. East African Trypanosomiasis Research Organization, Annual report, 1966, pp. 28-29.
Zwart, D., N.M. Perie, A. Keppler and E. Goedblood. 1973. A comparison of methods for the diagnosis of trypanosomiasis in East African domestic ruminants. Trop. Anim. Hlth Prod. 5: 79-87